Flu Vaccination Coding and Billing for 2022 – 2023
 November 9, 2022
November 9, 2022The Food and Drug Administration (FDA) Vaccines, and Related Biologic Products Advisory Committee selected vaccine strains for the 2022–2023 influenza vaccine. Also, the World Health Organization recommended the Northern Hemisphere as the basis for the 2022-2023 influenza vaccine composition.
Want more details? Visit the Centers for Disease Control (CDC) Flu Season web page for more information about the flu vaccines for 2022-2023.
Billing/Reporting Influenza Vaccines for Medicaid Beneficiaries
Depending on the age of the beneficiaries and vaccine formulation, the vaccine codes listed below may either be reported (with no charge) or billed (with a charge as usual and customary). The tables also show the administration codes that may be billed, depending on the beneficiaries’ age and vaccine(s) provided to them.
Vaccine CPT Codes to Report
Source: North Carolina Department of Health and Human Services (NCDDHS).
Table 1. A list of influenza billing codes for Medicaid beneficiaries under 19 years of age who receive the VFC influenza vaccine. The reporting of these codes results in a 0.00 dollar amount.
Providers should use the NDC on the actual vial used for administration listed at the bottom of this bulletin when processing claims.
Important Note: In the Health Check Billing Guide, you can find specific information about billing immunization administration codes for Health Check beneficiaries.
Source: North Carolina Department of Health and Human Services (NCDDHS)
Table 2: Influenza Billing Codes for Medicaid Beneficiaries 19 to 21 Years of Age
Providers should use the following codes to bill for influenza vaccines purchased and administered for Medicaid beneficiaries between the ages of 19 and 21.
Important Note: The VFC/NCIP provides influenza vaccines only to recipients between the ages of six months and 18 years old. However, those 19 years and older will not receive the influenza vaccine.
Vaccine CPT Codes to Report
Source: North Carolina Department of Health and Human Services (NCDDHS)
Administrative CPT Codes to Report
Source: North Carolina Department of Health and Human Services (NCDDHS)
Table 3: Influenza Billing Codes for Medicaid Beneficiaries 21 Years of Age and Older
The administrative CPT code 90472 will only be used if another vaccine is also administered along with the influenza vaccine. Moreover, it’s possible for providers to bill 90472 in more than one unit, if necessary.
In the event that beneficiaries 21 years of age and older purchase or receive an influenza vaccine, providers should use the following codes to bill Medicaid.
Important Note: Only VFC-age beneficiaries (6 months through 18 years of age) are eligible for influenza products under the VFC/NCIP. However, those 19 years and older will not receive the influenza vaccine.
Vaccine CPT Code to Report
Source: North Carolina Department of Health and Human Services (NCDDHS)
Administrative CPT Code(s) to Bill
Source: North Carolina Department of Health and Human Services (NCDDHS)
The administrative CPT code 90472 will only be used if another vaccine is also administered along with the influenza vaccine. Moreover, it’s possible for providers to bill 90472 in more than one unit, if necessary.
For beneficiaries 21 years or older receiving an influenza vaccine, an evaluation and management (E/M) code cannot be reimbursed to any provider on the same day that injection administration fee codes (e.g., 90471 or 90471 and +90472) are reimbursed.
Any healthcare provider cannot reimburse an evaluation and management (E/M) code for beneficiaries 21 years and older who are receiving influenza vaccinations on the same day as injection administration fee codes (e.g., 90471 and +90472). For billing a separately identifiable service, the provider must add modifier 25 to the E/M code.
Flu Vaccination Coding and Billing for 2022-2023
As shown in Table A, Medicare Part B payment allowances increased slightly for the 2022-2023 flu season.
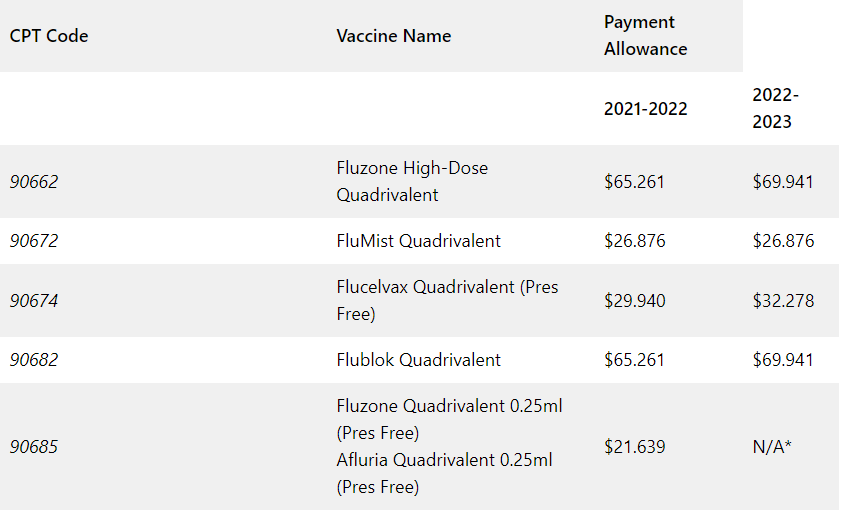
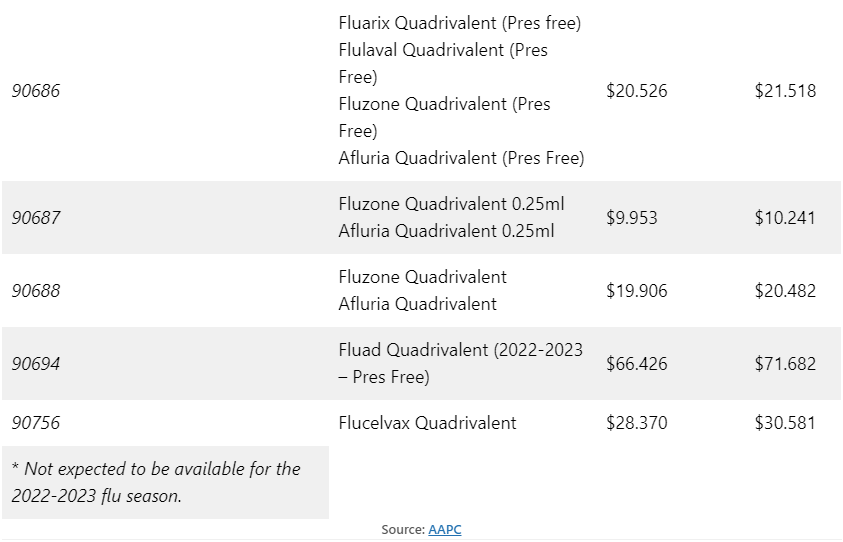
Table A: Comparison of CPT® code and Medicare Part B payment allowances for 2021-2022 and 2022—2023 flu seasons
Medicare Part B payment allowances are 95 percent of the average wholesale price (AWP) for influenza vaccines, except in cases where vaccines are provided in outpatient departments of hospitals, rural health clinics, or Federally Qualified Health Centers.
Important Note: In one calendar year, Medicare patients can receive two fully-covered flu vaccinations due to the annual flu season that runs from Aug. 1 to July 31.
When a patient receives a flu shot on Jan. 5, 2022, and again on Aug. 29, 2022, Medicare will pay for both vaccinations.
As usual, vaccinations against the influenza virus do not apply toward the annual Part B deductible or coinsurance amounts.
In accordance with Medicare guidelines, providers must report ICD-10-CM code Z23 Encounter for immunization together with administration code G0008 Administration of influenza virus vaccine.
While vaccine product pricing is updated on Aug. 1, G0008’s pricing is effective Jan. 1 through Dec. 31. Physicians can now download the 2022 Medicare Physician Fee Schedule (MPFS) payment rates file from the Centers for Medicare & Medicaid Services (CMS), which includes locality-adjusted payment rates for influenza, pneumococcal, and hepatitis B vaccine administration.
Important Safety Information for Physicians Administering Flu Vaccines
It is not recommended to administer Fluzone Quadrivalent, Flublok Quadrivalent, or Fluzone High-Dose Quadrivalent to anyone who has had a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccines (including egg protein for Fluzone Quadrivalent and Fluzone High-Dose Quadrivalent) or after a previous dose.
It is also not recommended to administer Fluzone Quadrivalent or Fluzone High-Dose Quadrivalent to anyone who has had an allergic reaction to any influenza vaccine in the past.
The Fluzone Quadrivalent injection site reactions in pain, tenderness, erythema, and swelling in children 6 months to 35 months of age. Acute injection-site reactions (pain, erythema, and swelling) are the most common in children 3 years through 8 years of age. Systemic adverse reactions include myalgia, malaise, and headaches. The most common solicited systemic adverse reactions in adults 18 years, and older were myalgia, headache, and malaise.
There was a higher frequency of pain at the injection site in adults 65 years of age and older, as well as headache, malaise, and myalgia in systemic adverse reactions to Fluzone High-Dose Quadrivalent.
It is possible that other adverse reactions will occur with Fluzone Quadrivalent, Flublok Quadrivalent, and Fluzone High-Dose Quadrivalent.
For all Fluzone Quadrivalent, Flublok Quadrivalent, and Fluzone High-Dose Quadrivalent vaccine products, please see the complete Prescribing Information. Also, you can check out the complete Patient Information for Fluzone Quadrivalent or Fluzone High-Dose Quadrivalent.
Influenza Vaccine Products for the 2022 – 2023 Influenza Season
Get ready for the 2022-2023 influenza season!
Using this article as a guide, we hope you will be able to administer flu vaccination codes more easily. For more information, you can also refer to this source.
Tap Into Our Expertise
At 5 Star Billing Billing Services Inc, we offer the highest level of performance for high-quality medical billing and coding. Save your money by outsourcing to a professional billing service.

